Background: The current standard of care (SoC) for newly diagnosed patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) is treatment with 1 of the 4 tyrosine kinase inhibitors (TKIs) approved for this use (first generation [1G] imatinib [IMA] and second generation [2G] bosutinib [BOS], dasatinib [DAS], and nilotinib [NIL]). Although TKIs have vastly improved treatment outcomes, pts with newly diagnosed CML-CP experience lack of efficacy and intolerance to available treatments. A large number of pts treated with IMA do not achieve major molecular response (MMR) and even fewer pts achieve deep molecular response, a key requirement to attempt treatment-free remission. Treatment with 2G TKIs results in larger proportions of pts achieving deep responses albeit still with a sizeable minority not reaching this goal. Also, many pts are intolerant to 2G TKIs, resulting in therapy switching, frequently multiple times. For pts who remain on treatment, even low-grade adverse events (AEs) may significantly impact quality of life (QoL) given the long-term nature of treatment. Improved treatments with high efficacy and improved tolerability are needed to limit therapy switching, reduce treatment discontinuation, maintain, or improve pt QoL, and allow pts to remain on treatment at an optimal dose for longer duration to achieve deep responses.
Asciminib is the first and only approved BCR::ABL1 inhibitor that works by specifically targeting the ABL myristoyl pocket (STAMP). This unique mechanism of action (MoA) has no confirmed overlapping mutation-driven resistance profile with ATP-competitive TKIs. Asciminib shows better selectivity compared to other TKIs, with no known off-target kinase-mediated effects due to its MoA; this translates into improved tolerability. In clinical trials (Hughes et al. N Engl J Med. 2019; Rea et al. Blood 2021), asciminib has demonstrated sustained efficacy and a favorable safety profile over time in pts with Philadelphia chromosome positive (Ph+) CML-CP previously treated with ≥2 TKIs as well as in pts with T315I mutation.
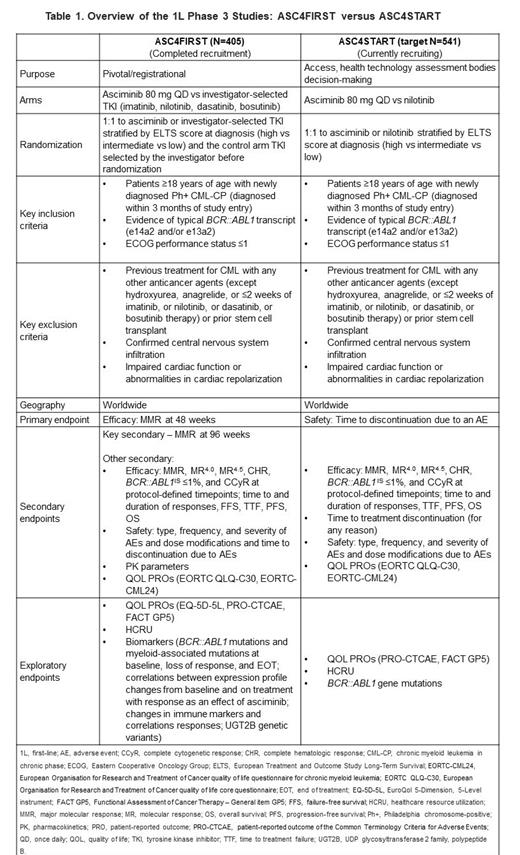
Asciminib as monotherapy and in combination with ATP-competitive TKIs is being studied as first-line (1L) treatment in CML. The ongoing ASC4FIRST (NCT04971226) study is investigating the efficacy and safety of asciminib 80 mg once daily (QD) monotherapy vs investigator-selected 1G or 2G TKI in adults with newly diagnosed Ph+ CML-CP ( Table 1). The primary endpoint of ASC4FIRST is MMR rate at 48 weeks. The ongoing ASC4START (NCT05456191) study is focused on pt-centric objectives, assessing the tolerability and efficacy of asciminib vs 2G TKI NIL in adults with newly diagnosed Ph+ CML-CP. The primary endpoint of ASC4START is time to treatment discontinuation due to an AE ( Table 1).
Current Status:
As of December 20, 2022, the ASC4FIRST study completed recruitment, with 405 pts enrolled worldwide. The end of study (EOS) will occur 5 years from the last pt first treatment in the study. Pts will continue to receive the assigned treatment until the EOS, or until premature discontinuation due to treatment failure, disease progression or intolerance, or due to investigator or participant decision.
Between 21 November 2022 and 25 July 2023, 180 pts have been screened for the ASC4START study, with 147 pts randomized to treatment (n=52 France, n=40 Germany, n=9 Bulgaria, n=7 Czechia, n=7 Hungary, n=6 Korea, n=6 Singapore, n=2 Slovakia, n=5 Argentina, n=3 Greece, n=2 each from Slovakia, Malaysia, and Oman, and n=1 each from the USA, Italy, Romania, UK, Jordan, and Canada). Recruitment is ongoing. Patients will be treated until approximately 64 discontinuations of either study treatment due to an AE have been recorded.
Conclusions:
With demonstrated efficacy and improved tolerability compared to ATP-binding TKIs, asciminib has the potential to become the therapy of choice for CML in 1L, enabling newly diagnosed patients to remain on therapy with improved QoL and overall survival, and reducing the need for therapy switching, ultimately allowing patients to achieve optimal milestone responses and reach treatment goals. Findings from these two trials will provide comprehensive evidenceon the use of asciminib as 1L therapy for newly diagnosed pts with Ph+ CML-CP.
Disclosures
Hochhaus:Novartis: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Pfizer: Research Funding; Incyte: Research Funding. Cortes:Gilead: Consultancy; Biopath Holdings: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Research Funding; Forma Therapuetic: Consultancy; Novartis: Consultancy, Research Funding; Takeda: Consultancy, Honoraria; Abbvie: Consultancy, Research Funding. Takahashi:Mochida Pharma: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Otsuka Pharmacuetical: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Asahi-Kasei: Research Funding; Astellas pharma: Other: Commissioned research and joint research , Research Funding. Larson:Bristol Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Issa:Merck: Research Funding; Novartis: Consultancy, Research Funding; Kura Oncology: Consultancy, Research Funding; Syndax: Research Funding; NuProbe: Consultancy; Celgene: Research Funding. Bombaci:Novartis: Consultancy, Other: Funding to affiliated organisation; CML Advocates Network: Membership on an entity's Board of Directors or advisory committees, Other: Funding to affiliated organisation; MPN Advocates Network: Membership on an entity's Board of Directors or advisory committees, Other: Funding to affiliated organisation; AIL: Membership on an entity's Board of Directors or advisory committees, Other: Funding to affiliated organisation; CLL Advocates Network: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Funding to affiliated organisation. Saussele:Novartis: Consultancy, Research Funding; BMS: Research Funding; Incyte: Research Funding; Pfizer: Consultancy; Roche: Consultancy. Mahon:Pfizer: Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria. Brümmendorf:Merck: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Speakers Bureau. Kapoor:Novartis: Current Employment. McCulloch:Novartis: Current Employment. Schuld:Novartis: Current Employment. Hughes:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Terns Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Enliven: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal